


Introduction to Apoptosis (Part Four)
By: Lionel Perez Valenzuela
1) The apoptosis triggered by internal signals: the intrinsic pathway or mitochondrial pathway
Key Concepts in this way:- The mitochondrion is a crucial point of control in the induction of apoptosis.
- Apoptosis is an active and controlled process that requires the synthesis of RNA and protein by the cell dying.
a) The caspases: initiator and effector of apoptosis:
Caspases are proteases that have an amino acid cysteine \u200b\u200bin the active site, so are cysteine proteases. Any mutation affecting this amino acid to stop the protease inactive.
Caspases are named after more than a dozen of the same family proteases. The term refers to the spot where they cut (C) proteins after a amino acid aspartate (Asp), Caspases hence the name (other authors only refer to the term derives from caspase cysteine \u200b\u200baspartate proteases ).
These proteins can be divided into two groups initiator caspases (like caspase 9), which are activators of effector caspases (caspase 3).
The effector caspases are ultimately responsible to execute the cell, cleaving the protein that causes cell death. Because caspases can cause apoptosis, are closely regulated proteins. But then again, there needs to be activated if needed quickly. How is this done? In the first term does not regulate its transcription (caspases are present in virtually all cell types), but its activation to post-transcriptional level. Caspases are synthesized as pro-caspase immature. Ie need to be processed (cleaved) to become active.
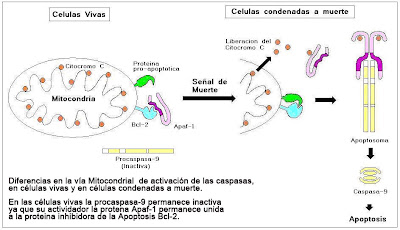
b) Cytochrome c and Apaf-1, caspase 9 activators
Cytochrome c is a component of the respiratory chain, which acts as a proapoptotic factor when released into the cytoplasm. The release of cytochrome c, is preceded by changes in mitochondrial membrane permeability. Once released into citoplasama cytochrome c binds to the protein Apaf-1 (activating factor-1, apoptotic protease). This complex cytochrome c, Apaf-1 and oligomerize ATP and binds to the immature pro-caspase 9 activities, forms the apoptosome. The pro-active caspase-9 once it cleave to each other, forming the mature caspase (a tetramer with four subunits, two large and two small).
mature caspase 9 then initiates a proteolytic cascade that expands (amplification), activating other effector caspases (eg caspase 3), eventually leading to cellular changes characteristic of apoptosis.
c) Summary of the track
healthy cells, expressed on the surface of the outer mitochondrial membrane protein Bcl-2 . This protein in turn serves to anchor the protein Apaf-1 (activation factor-1 protease apoptotic). We can say that Bcl-2 protein is a protein inhibitor of apoptosis, because it holds to the Apaf-1
When generating internal damage cellular signals (eg free radical), the protein Bcl-2 protein released into the Apaf-1. A related protein called Bcl-2 Bax forms a pore and enters the mitochondrial membrane, causing the exit of cytochrome c (a component of the respiratory chain). Then, the protein is pro-apoptotic Bax, allowing exit of cytochrome c binding to Apaf-1.
released proteins, cytochrome c (plus the ATP required for activation) and Apaf-1 bind to the protease caspase 9. The result of the union of these three proteins cytochrome c, Apaf-1 and caspase 9, resulting in the formation of the complex called apoptosome.
The apoptosome begins proteolytic cascade that leads ultimately to the digestion of cytoplasmic structural proteins, structural proteins of the nucleus, degradation of the chromosomes and cell phagocytosis .
0 comments:
Post a Comment